Many commercial buildings are constructed with large structural steel and metallic components. Metals are fantastic for building and they have the ability to create long-lasting, extremely durable structures. The kryptonite to any metal structure (steel in particular) however is corrosion.
What Exactly is Corrosion?
It begins small, with barely noticeable pitting that does not raise any red flags. The issue with corrosion, in general, is the ability for that small visual effect to spread beneath the surface where corrosive elements eat away at the steel. It spreads like cancer, compounding the damage when not addressed and stopped.
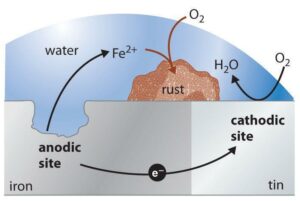
Corrosion is caused by contact and exposure to moisture, chemicals, salts, and oxygen. The scientific process is electrochemical, where anodes exchange electrons to cathodes via a metallic pathway. When the anode loses electrons, it becomes corroded metal and as the process continues, it essentially eats away at the metal.
 Left unchecked, the process of corrosion will continue until the metal is structurally weakened and unsound. Given enough time, it will eat away at the metal almost entirely. Think about an old vehicle left to the elements. Steel rusts, leaving gaping holes in the once solid structure.
Left unchecked, the process of corrosion will continue until the metal is structurally weakened and unsound. Given enough time, it will eat away at the metal almost entirely. Think about an old vehicle left to the elements. Steel rusts, leaving gaping holes in the once solid structure.
Localized vs. General Corrosion
The two primary types of corrosion are divided between localized and general and both should be addressed and repaired ASAP. Each has different identifiers but both share the quality of putting the structure at risk.
Generalized corrosion happens over a larger or general area and is often the result of iron oxide produced when the steel is initially rolled. Often referred to as mill scale, it has a bluish tint and must be removed before industrial coatings are applied. Otherwise, the iron oxide leads to corrosion beneath the coatings and causes bubbling across the broad steel surface.
Localized corrosion is very exacting and commonly found in crevices or identified by pitting. Imperfect coatings and coverage cause pinholes which allow the metal surface to be exposed to the environment allowing the metallic pathway to be completed and corrosion to occur. When water or chemicals are allowed to penetrate the coating, the corrosion spreads beneath the surface and can wreak havoc on the metal.
Responding to Corrosion
Once the corrosion begins, it will spread when left untreated. It’s not uncommon to quote a restoration project only to require a new inspection and quote because ownership decided to wait a year. The corrosion will continue spreading, making it imperative to address the issue immediately.
More importantly, it’s more cost-effective and easier to treat the earlier stages of corrosion. In extreme cases, the structural steel can even reach a point where replacement is necessary. Be diligent about inspections, respond early and you will save in the long run by stopping corrosion before it spreads.
Better yet, implement a maintenance program that includes regular inspections and restoration work to mitigate corrosion. These maintenance best practices for industrial tank corrosion are a great place to start.
How Coatings Protect Against Corrosion
Industrial coatings play a critical role in protecting steel structures against corrosion. A number of different coatings exist for specific types of structures and the elements they are commonly needing protection against. Waterproofing membranes are not uncommon as water and even moisture in the air in humid regions is a leading cause of corrosion.
The primary goal of most coatings is to shield the steel from contact with salts, chemicals and moisture. Stripe coating in particular is done ahead of the full coating to cover welds, joints, crevices and other vulnerable areas. After application, industrial coatings require regular inspections and occasional renewals to maintain the layer of protection that prevents water damage and contact with corrosive elements.
When two dissimilar metals are placed in constant contact, the more active metal corrodes, this is called galvanic corrosion. Layering a more active metal like zinc over carbon steel creates cathodic protection because the zinc corrodes and protects the steel beneath. Cathodic protection is extremely effective at protecting steel and extending its lifespan and it all works because the corrosion is isolated to the zinc coating.
Now you know how corrosion works and the urgency required to stop it from causing irreparable damage. Start the inspection process and tackle your corrosion woes head-on to protect your structure and investment.
Protect your steel structures
Don’t put off inspections and repairs for corrosion. It’s easier and cheaper to restore steel structures immediately after identifying vulnerabilities and pitting. Get in touch with McGill Restoration to plan your steel restoration process today.



